Fungal spores harness physics to launch themselves
16 August 2023 2023-08-16 13:25Fungal spores harness physics to launch themselves

Fungal spores harness physics to launch themselves
Source: Duke University
Summary: More than a century ago, Reginald Buller discovered that a spherical drop of water that forms close to a spore is crucial to the spore’s dispersal. Now, using an ink jet printer and high speed cameras, researchers have uncovered the detailed mechanics of the way fungal spores have evolved to harness the power of merging water droplets to launch in a uniform manner.
Researchers from Duke University have uncovered the detailed mechanics of the way fungal spores have evolved to harness the power of merging water droplets to launch in a uniform manner.
Fungal spores grow on the ends of long, thin tethers called sterigmas. Once mature, the spores must break away and be transported to a new location to grow. Some spores rely on animals or their own power to travel. Others — called ballistospores — are actively ejected from the surface of the parent organism. And in the case of some fungi, water droplets provide the liftoff.
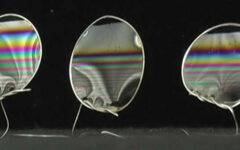
More than a century ago, Reginald Buller discovered that a spherical drop of water that forms close to a spore is crucial to the spore’s dispersal. Dubbed the “Buller drop,” its merging with another lens-shaped drop on the spore causes the spore to break away from its tether.
“The spores are launched with a massive amount of force in a specific direction, almost like a cannon,” said Chuan-Hua Chen, associate professor of mechanical engineering and materials science at Duke. “And the ballistospore cannon has evolved to shoot directly away from the fungus to give the spores the best chance of escape.”
While this phenomenon had been explained energetically, the detailed mechanisms — particularly the nearly uniform direction of the spores’ launches — have remained a mystery. In a paper published in the Journal of the Royal Society Interface on July 27, Chen and his colleagues use high-speed cameras and an inkjet printer to solve the riddle.
The major hurdle to uncovering the details of how water droplets launch these spores has been the speed of the action. While it takes several minutes for water droplets to grow large enough for takeoff, the event itself takes less than a microsecond.
“And unfortunately a microsecond is also the time resolution for most high speed cameras,” Chen said. “So while researchers made some progress in capturing the overall coalescence process, the detailed mechanism was still not clear.”
The problem was one of scale and timing, as the duration of the launch is proportional to the size of the Buller drop, which is tiny when it comes to fungal spores.
To sidestep this issue, Chen and his team constructed their own larger “spores” by cutting a polystyrene sphere into a spore-shaped particle and carefully orienting the model spore on a flat surface. Then they used an ink-jet printer to build a larger Buller drop directly next to their artificial spore. With the ability to precisely control the drop’s size, and thus its takeoff speed and timing, the team could catch the launch with high resolution.
When they looked at the film, the details of the launching mechanism became apparent. When the spherical Buller drop joins the second drop that is spread on the spore, the drops lose surface area and release surface energy, providing the momentum for the launch.
As the newly merged drop moves along the flat face of the spore, the drop motion quickly aligns with the orientation of the spore’s flat face. The merged drop exerts friction on the spore as it moves and pulls it away from the sterigma. The direction of launching is guided by the spore’s flat face, which is along the same direction as the slender sterigma.
“The release of energy is so rapid that it accelerates the entire system with a million Gs, but there’s so much air drag that the spore still only travels a few millimeters at most. That’s why it’s so important for the spores to shoot directly away from the fungus,” Chen said. “By explaining the mechanism underlying the nearly perfect launching directionality, our work has finally shed light on this century-old puzzle.”
This work was supported by the National Science Foundation (CBET-1236373, DMR-1121107) and the National Institute of Health (T32-GM008555). Additional support came from the National Science Foundation (IOS-1439850), the Human Frontier Science Program and the Natural Sciences and Engineering Research Council of Canada.